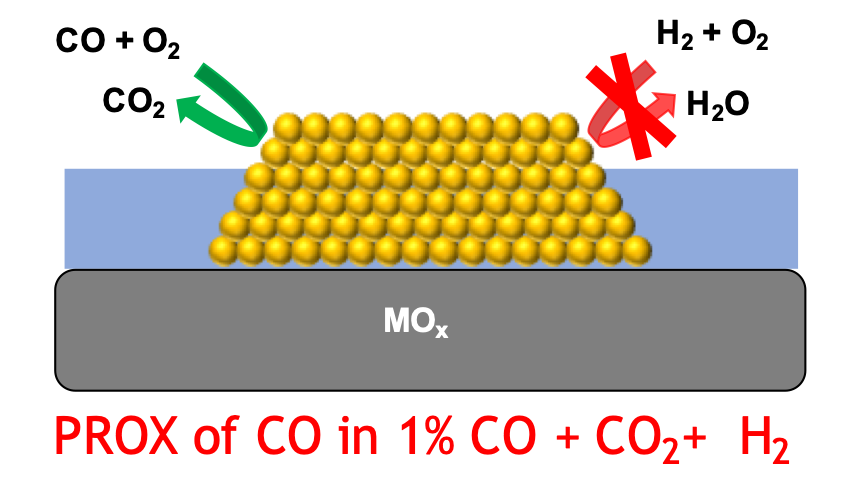
Hydrogen (H2), being a clean fuel is touted to be the replacement for carbon based fuels in an effort to reduce greenhouse gas emissions. H2 is primarily produced through steam reforming of natural gas followed by water-gas shift treatment. The CO content in the effluent H2 stream from the water-gas shift reactor must be lowered from 1% to 0.2 ppm to prevent CO poisoning in applications such as fuel cells with Pt electrodes. Preferential oxidation (PROX) of CO with O2 in hydrogen rich streams is more energy efficient solution than the currently used CO methanation. The motivation behind our research stems from Haruta’s discovery of activity of gold nanoparticles supported on metal oxides towards CO oxidation. This is surprising given the long standing belief that gold was chemically inert. Water was shown by our group to enhance CO oxidation rates through O2 activation to active OOH* intermediate on Au/TiO2. Currently, we are implementing the use of water as a strategy to improve the selectivity of supported gold catalysts towards PROX of CO in water-gas shift streams. We use density functional theory, FTIR spectroscopy and kinetic experiments in collaboration with Dr. Chandler’s group at Trinity University to understand CO and H2 oxidation on the model Au/TiO2 catalyst. We have recently discovered the heterolytic H2 activation at the metal-support interface (MSI) of Au/TiO2 using DFT calculations and supported the discovery with H-D exchange experiments using FTIR spectroscopy. Water was found to poison the active interface sites and also kinetically hinder the H2 activation at the MSI, thus enhancing the selectivity for CO oxidation. We are also working on exploiting this unique chemistry of MSI sites to activate more challenging molecules such as methane, which has applications in upgrading natural gas to value added chemicals.
Publications:
- Saavedra, J., Doan, H. A., Pursell, C. J., Grabow, L. C. & Chandler, B. D. The critical role of water at the gold-titania interface in catalytic CO oxidation. Science (80-. ). 345, 1599–1602 (2014).
- Tran, H.-V., Doan, H. A., Chandler, B. D. & Grabow, L. C. Water-assisted oxygen activation during selective oxidation reactions. Curr. Opin. Chem. Eng. 13, 100–108 (2016).
- Whittaker, T., Kumar, K. B. S., Peterson, C., Pollock, M. N., Grabow, L. C. & Chandler, B. D. H 2 Oxidation over Supported Au Nanoparticle Catalysts: Evidence for Heterolytic H 2 Activation at the Metal–Support Interface. J. Am. Chem. Soc. 140, 16469–16487 (2018).
- Bruno, J. E., Sravan Kumar, K. B., Dwarica, N. S., Hüther, A., Chen, Z., Guzman, C. S., Hand, E. R., Moore, W. C., Rioux, R. M., Grabow, L. C. & Chandler, B. D. On the Limited Role of Electronic Support Effects in Selective Alkyne Hydrogenation: A Kinetic Study of Au/MO x Catalysts Prepared from Oleylamine‐Capped Colloidal Nanoparticles. ChemCatChem 11, 1650–1664 (2019).
- Chandler, B. D., Kendell, S., Doan, H., Korkosz, R., Grabow, L. C. & Pursell, C. J. NaBr Poisoning of Au/TiO2 Catalysts: Effects on Kinetics, Poisoning Mechanism, and Estimation of the Number of Catalytic Active Sites. ACS Catal. 2, 684–694 (2012).
- Doan, H. A., Sharma, M. K., Epling, W. S. & Grabow, L. C. From Active-Site Models to Real Catalysts: Importance of the Material Gap in the Design of Pd Catalysts for Methane Oxidation. ChemCatChem 9, 1594–1600 (2017).